Neutralizing Acids and Bases – Quick Tips
Spills of hazardous substances must be absorbed, neutralized or controlled at the time of the incident in order to maintain workplace safety. Acid and base neutralization helps make spilled materials safer to handle and helps decrease the cost of disposal.
How Do You Know If a Solution Is Acidic or Basic?
The best way to determine if a material is acidic or basic is to measure its pH. This can be accomplished with pH paper, chemical indicators or pH meters. The pH scale measures from 0 to 14. Chemicals with a pH of 0 to 3 are considered strong acids. Chemicals with a pH of 12 to 14 are considered strong bases. To be considered neutral, a chemical must have a pH of 7.
Acids typically will have a sour taste and a pH of less than 7. There are two types of acids: mineral (inorganic) acids such as sulfuric, hydrochloric or nitric and carboxylic (organic) acids such as formic or acetic. To neutralize acids, a weak base is used.
Bases have a bitter or astringent taste and a pH greater than 7. Common bases are sodium hydroxide, potassium hydroxide and ammonium hydroxide. Bases are neutralized by using a weak acid.
Products for Acid and Base Neutralization
There are many different products available that aid in the neutralization of acids and bases. They can be as simple as a bag of citric acid or sodium bicarbonate (baking soda), or as complex as a solidifier and a neutralizer combined. Some of the major considerations in selecting the best method of neutralization are safety (mixing chemicals is always a potentially dangerous process), cost and convenience.
When neutralization occurs, the acid and base react, forming water, salt and heat. If the acid and base are both very strong (such as concentrated hydrochloric acid and concentrated sodium hydroxide), a violent reaction will occur. This is why most neutralizers are very weak — to help keep the reaction at a slow pace and lessen the evolution of heat and gas.
Most neutralizer manufacturers provide an estimated volume of acid/base that their product will neutralize. It can take a large amount of the product to neutralize an acid or base, especially if it is concentrated. Some acid and base neutralizers have a built-in color indicator to let you know when the spill is neutralized. Other neutralizers require you to check the pH with pH paper or a pH meter to monitor the neutralization process. Some neutralizers also solidify the spill as they neutralize, to help make the spill easier to clean up. See the comparison charts below for examples of treatment ratios for some common neutralizers for acids and bases.
Chart A
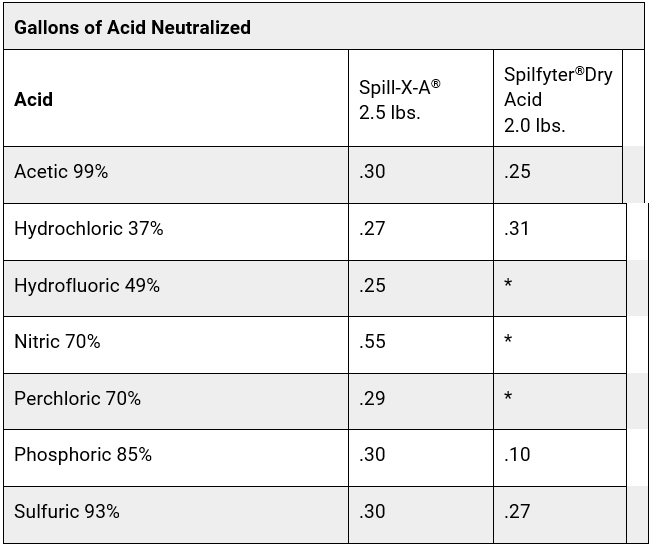
*Not Applicable
Chart B
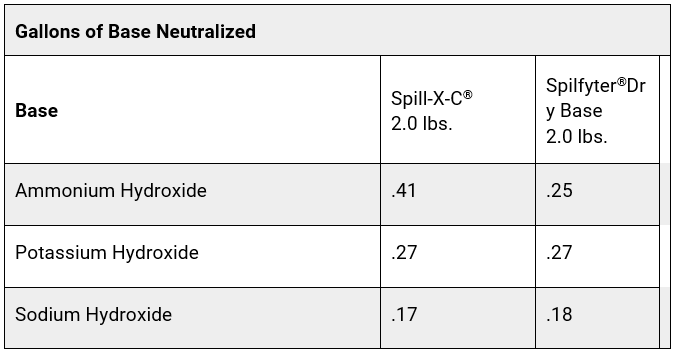
Sources
ANSUL Spill X
NPS Corporation
Source: Grainger Know How – https://www.grainger.com/know-how